P2B001 Clinical Evidence
P2B001 efficacy was demonstrated in a 12-week multi-center, multinational, randomized, double-blind, double-dummy, active-controlled, parallel group study, designed to compare P2B001 to its individual components (pramipexole 0.6mg ER [PPX-ER, rasagiline 0.75mg ER [RAS-ER]) and to a calibration arm of marketed pramipexole ER (PramiER) titrated to optimal dose.
It enrolled 544 treatment-naïve, early-stage Parkinson’s patients (< 3 years from diagnosis), aged 35-80 years, across 70 centers in US, Europe and Canada.
Patients were randomized to one of four treatment arms: P2B001 Extended Release capsules, pramipexole 0.6mg ER capsule (PPX-ER, a component of P2B001) ; rasagiline 0.75mg ER capsule (RAS-ER, a component of P2B001); and the currently marketed pramipexole ER (PramiER) titrated to an optimal dose for each individual patient (1.5-4.5mg). For more information on the trial, refer to ClinicalTrials.gov Identifier: NCT03329508
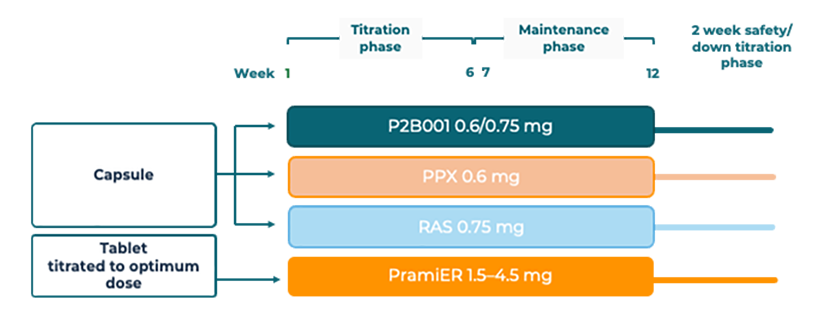
*Patients either received active capsules and placebo tablets, or placebo capsules and active tablets
The study completed successfully and met its primary and key secondary endpoints. P2B001 treatment improved symptomatic management, comparable to higher doses of marketed PramiER (mean dose 3.2mg), yet with fewer dopaminergic side effects and significantly less daytime sleepiness. P2B001’s efficacy was also significantly superior to that from each of its components.
This data was further supported by the Phase IIb study, conducted in 149 early PD patients in the US and Israel, where P2B001 was superior to a placebo control in all endpoints, including UPDRS, various Quality of Life scales, and in the Clinical Global Impression of Disease Severity Scale (P2B001 treatment resulted in a significant and clinically meaningful change).
P2B001 Clinical Benefits in a Nutshell
|
P2B001 Improved PD Motor Symptoms
P2B001 was superior to its individual components PPX-ER 0.6 and RAS-ER 0.75 and comparable to marketed higher dose PramiER (mean dose 3.2mg) in change from baseline to week 12 on the UPDRS total score (-7.98±0.60 points vs -8.35±0.86 points for P2B001 and PramiER , respectively p=0.7197)
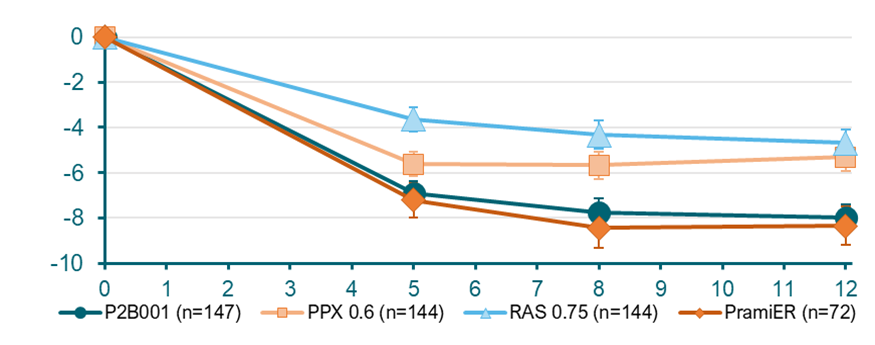
P2B001 was also found to Improve Activity of Daily Living (UPDRS Part II) significantly better than its individual components PPX-ER 0.6 and RAS-ER 0.75 and comparable to marketed higher dose PramiER (mean dose 3.2mg).
References:
- Olanow CW et al. A randomized trial of a low-dose Rasagiline and Pramipexole combination (P2B001) in early Parkinson’s disease. Mov Disord. 2017 May;32(5):783-789. doi: 10.1002/mds.26941.
- Hauser RA et al. P2B001 (Extended Release Pramipexole and Rasagiline): A New Treatment Option in Development for Parkinson’s Disease. Adv Ther. 2022 May;39(5):1881-1894. doi: 10.1007/s12325-022-02097-2.
- Olanow WG. Efficacy and safety of P2B001 in the management of early Parkinson’s disease. Results from a phase 3, randomized, double-blind, double-dummy controlled trial. Presented at: AAN Annual Meeting; April 2-7, 2022; Seattle, WA, and virtual. Abstract 011.
