P2B001 Safety
The safety and tolerability profile of P2B001 has been well characterized during the multi-national Phase III clinical trial program. Overall tolerability was high and only 8.7% treated with P2B001 did not complete the treatment, out of which 5.3% withdrew due to Adverse Events (AEs).
P2B001 had significantly reduced daytime sleepiness compared with PramiER as shown by change in ESS (Epworth Sleepiness Scale) during 12 weeks of treatment.
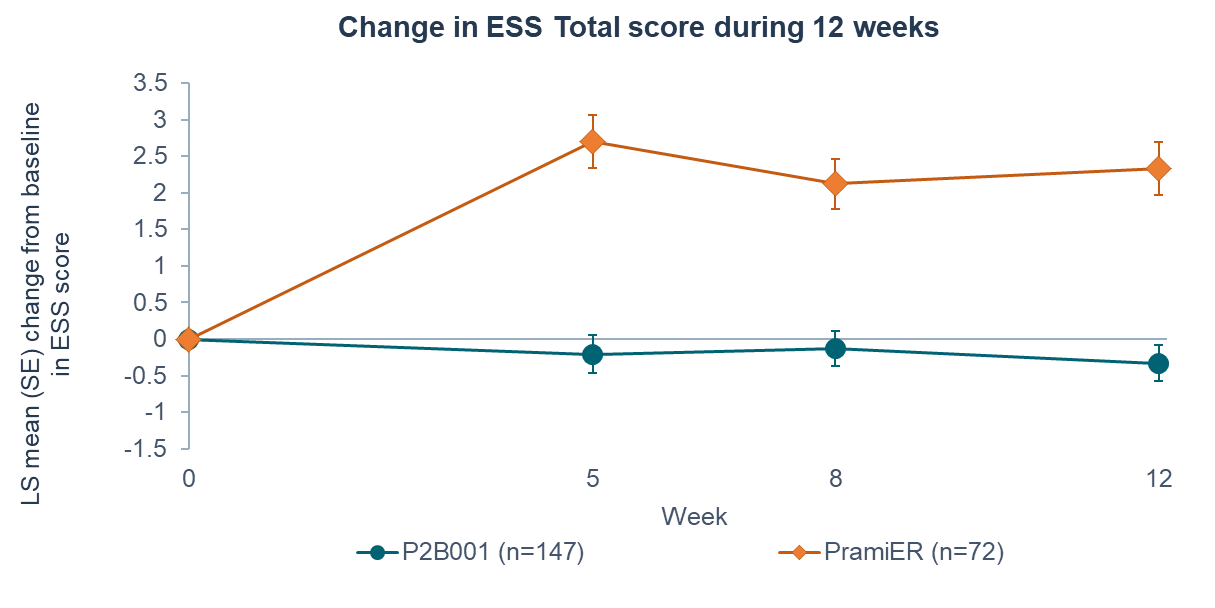
Furthermore, significantly less subjects shifted to extensive daytime sleepiness (from ≤10 to >10 ESS score) with P2B001 compared to PramiER (8.5% vs. 35.7%, p<0.0001)
Adverse Events
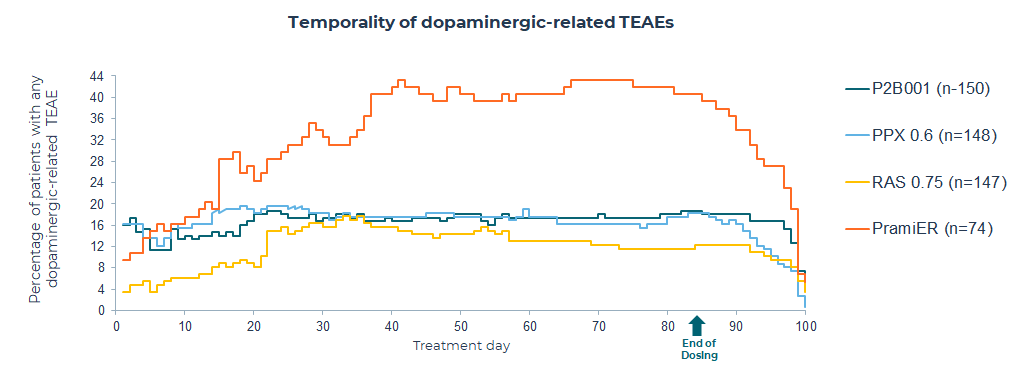
More than 98% of the treatment-emergent adverse events (TEAEs) were mild or moderate in severity. Phase 3 results demonstrated fewer treatment-realted adverse events with P2B001 vs. pramipexole-ER throughout the entire study. For more information on P2B001 safety, press here.
References:
- Olanow CW et al. A randomized trial of a low-dose Rasagiline and Pramipexole combination (P2B001) in early Parkinson’s disease. Mov Disord. 2017 May;32(5):783-789. doi: 10.1002/mds.26941.
- Hauser RA et al. P2B001 (Extended Release Pramipexole and Rasagiline): A New Treatment Option in Development for Parkinson’s Disease. Adv Ther. 2022 May;39(5):1881-1894. doi: 10.1007/s12325-022-02097-2.
- Olanow WG. Efficacy and safety of P2B001 in the management of early Parkinson’s disease. Results from a phase 3, randomized, double-blind, double-dummy controlled trial. Presented at: AAN Annual Meeting; April 2-7, 2022; Seattle, WA, and virtual. Abstract 011.
